Researchers from the Swiss Nanoscience Institute, the University of Basel, and the University Hospital Basel have developed a new nanotechnology based method to rapidly detect genetic mutations in patients with malignant melanoma. Findings from the new study, “Fast Diagnostics of BRAF Mutations in Biopsies from Malignant Melanoma”, were published recently in Nano Letters.
The American Skin Cancer Foundation estimates that more people develop skin cancer than breast, prostate, lung, and colon cancer combined. While malignant melanoma accounts for only about 5 percent of skin cancers, these are the most severe cases and often result in death.
Interestingly, around half of all patients who develop malignant melanoma exhibit a particular genetic mutation in the BRAF gene (B gene for Rapid Acceleration of Fibrosarcoma) that leads to uncontrolled cell proliferation. Thankfully, there are currently a few drugs that exploit these specific mutations and fight the cancer, significantly extending patients' life expectancy. However, the compounds work only if the corresponding genetic variation is present. Where it is not, the drugs give rise to severe side effects and provide little help in combating the disease.
Study co-author Katharina Glatz, Ph.D., of the Institute of Pathology at University Hospital Basel added that “it is, therefore, essential that we can identify the mutations reliably in tissue samples. That is the only way of ensuring that patients get the right treatment and successful outcomes.”
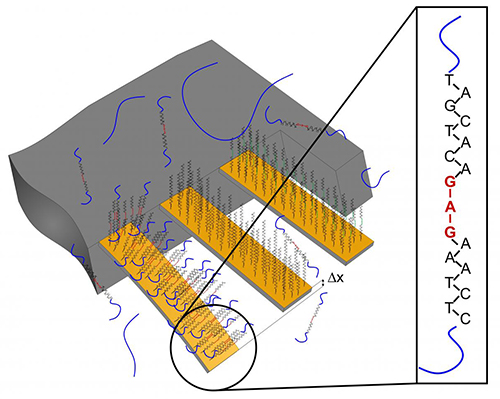
For the current study, the researchers employed tiny cantilevers that were coated in different ways. Some of them carried a recognition sequence for the BRAF mutation the researchers were targeting.
“We completed a preliminary clinical trial applying cantilever array sensors to demonstrate identification of a BRAFV600E single-point mutation using total RNA obtained from biopsies of metastatic melanoma of diverse sources (surgical material either frozen or fixated with formalin and embedded in paraffin),” the authors wrote.
RNA from patient tissue samples was isolated and applied to the cantilevers. If the genetic change was present, the patient's RNA would bind to the recognition sequence on the cantilever. The resulting surface stress would lead to a bending of the cantilever, which can be measured. If the mutation is absent from the RNA sample, this bending does not occur—in other words, only a specific binding produces a signal. The advantage of using nanocantilevers is that no time-consuming procedures are needed, and it takes less than a day to move from performing the biopsy to diagnosis.
“The method is faster than the standard Sanger or pyrosequencing methods and comparably sensitive as next-generation sequencing,” the authors penned. “Processing time from biopsy to diagnosis is below one day and does not require PCR amplification, sequencing, and labels.”
The Swiss researchers were able to demonstrate that nanomechanical microcantilevers—originally designed for use in atomic force microscopy—can identify mutations in complex mixtures of total RNA isolated from tissue samples.
“Thirty years ago, we weren't able to foresee that our technology might one day be used in hospital for personalized medicine—from the bench to the bedside, as it were,” noted senior study investigator Christoph Gerber, Ph.D., director of scientific communication at NCCR Nanoscale Science and professor in the department of physics at the University of Basel.













