Royal Philips has announced a pair of technologies aimed at helping fight cancer—including the launch of the latest edition of its IntelliSpace Portal advanced visualization and quantification platform, and the development of a combined liquid biopsy/imaging approach for which a consortium led by the company has won a €6.3 million ($7.5 million) grant from the European Union.
IntelliSpace Portal 10 is designed to support the reading and follow-up of complex oncology cases by clinicians and radiologists through a single, vendor-agnostic platform that offers tools that can be used across the continuum of oncology care, Philips said.
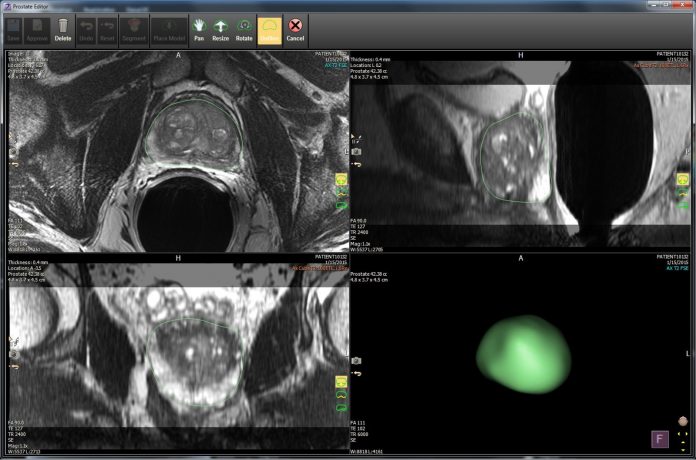
IntelliSpace Portal 10 also includes a new 3D modeling application and has been expanded with the inclusion of the DynaCAD Prostate and Breast solutions, through integration with Philips’ Invivo business, which focuses on advanced clinical visualization systems and MRI-compatible interventional devices.
Invivo’s DynaCAD Prostate and Breast—both advanced image analysis solutions designed to help reduce processing time to assist with enhanced disease management—will be directly integrated into IntelliSpace Portal 10. According to Philips, DynaCAD Prostate and Breast feature flexible workspaces with custom hanging protocols, image post-processing, lesion segmentation, standardized and structured reporting and interventional biopsy planning.
“This new edition marks Philips' ability to provide one comprehensive solution to answer our customers' needs, such as clinical improvements and increased efficiencies, especially now with the integration of two important clinical domains of breast and prostate cancer,” Yair Briman, business leader, healthcare informatics at Philips, said in a statement.
Among users of IntelliSpace Portal 10 disclosed by Philips is Phoenix Children's Hospital, which according to the company leverages the solution so that clinicians can use the 3D modeling features for clinical applications to help improve patient care. Philips is enabling users of the platform to create 3D models to help physicians understand hard-to-visualize patient anatomy through recently-signed agreements with two developers of 3D printing technology, 3D Systems and Stratasys.
“The 3D modeling application of IntelliSpace Portal 10 has been instrumental in receiving a more accurate picture of the details of anatomy. This is especially true as we prepare for complex procedures by determining the tumor's exact size and seeing subtle changes over time; informing the most viable treatment choice,” added Dianna Bardo, director, body MR, and co-director of the 3D Innovation Lab at Phoenix Children's.
Philips rolled out IntelliSpace Portal 10 yesterday during the Radiological Society of North America (RSNA) Annual Meeting, being held through Friday in Chicago.
Three days earlier, the company trumpeted the €6.3 million ($7.5 million) grant from the EU’s Horizon 2020 research and innovation program to a consortium led by Philips. The grant is designed to fund the consortium’s four-year research project to develop an integrated approach for personalized early-stage cancer treatment that increases the accuracy of both genetic and functional characterization of primary breast and rectal cancer.
The four-year research project, ‘Liquid biopsies and IMAging for improved cancer care’ (LIMA), will combine liquid biopsy with magnetic resonance imaging (MRI). The clinical research studies will be carried out at the Institut National de la Sante et de la Recherche Medicale (INSERM) in France, and the University Medical Center Universitair Medisch Centrum Utrecht (UMC Utrecht) in the Netherlands.
“This project will allow us to build methods to look inside the body in much more detail, which has the potential to improve the treatment we provide and make a significant positive impact on the lives of our patients,” stated Kenneth Gilhuijs, associate professor and research coordinator at UMC Utrecht.
Added Hans Hofstraat, innovation program manager at Philips: “In collaboration with our partners we will combine a range of liquid biopsy technologies, which give us more detailed molecular information, with advanced MRI techniques, which could enable us to better understand the impact of treatment at an early stage.”
Joining Philips, INSERM, and UMC Utrecht in the consortium are several small and medium-sized enterprises that have developed innovative technologies for liquid biopsies—including three providers of technologies for analyzing ct-DNA, Agena Bioscience, DiaDx, and Stilla Technologies; one company focused on circulating tumor cells (CTC) isolation, ANGLE plc; and ALS Automation Lab Solutions, which specializes in single CTC detection and selection.











