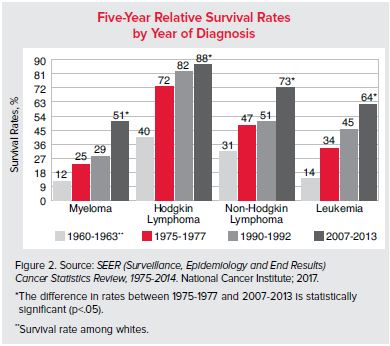
Statistics can be a great tool for analyzing datasets and it can also be a sobering means to provide a stark reality. For instance, recent data suggests that approximately every three minutes one person in the United States is diagnosed with some form of blood cancer. While this statistic shows that we still have a long way to go in combating cancers such as leukemia, there is light at the end of the tunnel, as data from the National Cancer Institute shows that five-year survival rates for leukemia and other blood cancers have increased dramatically over the last 60 years.
 To improve survival rates and aid scientific research for one of the most severe forms of leukemia— acute myelogenous leukemia (AML)—researchers at the University of Texas at San Antonio (UTSA) and the University of Texas MD Anderson Cancer Center created an online atlas to identify and classify protein signatures present at AML diagnosis. Sadly, only about one in four people diagnosed with AML survive five years after the initial diagnosis.
To improve survival rates and aid scientific research for one of the most severe forms of leukemia— acute myelogenous leukemia (AML)—researchers at the University of Texas at San Antonio (UTSA) and the University of Texas MD Anderson Cancer Center created an online atlas to identify and classify protein signatures present at AML diagnosis. Sadly, only about one in four people diagnosed with AML survive five years after the initial diagnosis.
The new protein classifications will help researchers and clinicians recommend better treatment and personalized medicine for patients suffering from this aggressive cancer, which occurs in the blood and bone marrow. Findings from the new study were released today in Nature Biomedical Engineering through an article titled “A quantitative analysis of heterogeneities and hallmarks in acute myelogenous leukemia.”
In the current study, the researchers examined the genetic, epigenetic, and environmental diversity that occurs in cancerous cells due to AML. Analyzing proteomic screens of 205 patient biopsies obtained at MD Anderson Cancer Center, the investigators developed a new computational method called MetaGalaxy to categorize the protein signatures into 154 different patterns based on their cellular functions and pathways.
“Here, we provide a comprehensive quantitative understanding of AML proteomic heterogeneities and hallmarks by using the AML Proteome Atlas, a proteomics database that we have newly derived from MetaGalaxy analyses, for the proteomic profiling of 205 patients with AML and 111 leukemia cell lines,” the authors wrote. “The analysis of the dataset revealed 154 functional patterns based on common molecular pathways, 11 constellations of correlated functional patterns, and 13 signatures that stratify the outcomes of patients. We find limited overlap between proteomics data and both cytogenetics and genetic mutations. Moreover, leukemia cell lines show limited proteomic similarities with cells from patients with AML, suggesting that a deeper focus on patient-derived samples is needed to gain disease-relevant insights.”
By approaching this challenge through the unique lens of developing a quantitative map for each leukemia patient from protein expression in their blood and bone marrow, rather than the standard lens of qualitative metrics and genetic risks alone, researchers will be able to more precisely categorize patients into risk groups and better predict their treatment outcomes.
To better understand the AML hallmarks at the proteomic level and to share the results of their work with other researchers, the scientists built a web portal known as the Leukemia Proteome Atlas. The online portal gives oncologists and cancer scientists the tools they need to investigate AML protein expression patterns from one patient to the next. It also provides investigators around the world with leads for new leukemia research and new computational tools.
Since many genetic mutations cannot be targeted, the proteomic profiling and target identification process used in this research study will accelerate the identification of therapeutic targets. It also propels researchers much closer to the development of personalized combination therapies for patients based on their unique protein signatures.
“Acute myelogenous leukemia presents as a cancer so heterogeneous that it is often described as not one, but a collection of diseases,” stated co-senior study investigator Amina Qutub, an associate professor in the UTSA department of biomedical engineering. “To decipher the clues found in proteins from blood and bone marrow of leukemia patients, we developed MetaGalaxy to identify molecular hallmarks of leukemia. These hallmarks are analogous to the way constellations guide navigation of the stars: they provide a map to protein changes for leukemia. Our ‘hallmark’ predictions are being experimentally tested through drug screens and can be ‘programmed’ into cells through synthetic manipulation of proteins.”
Qutub concluded that “a next step to bring this work to the clinic and impact patient care is testing whether these signatures lead to the aggressive growth or resistance to chemotherapy observed in leukemia patients. At the same time, to rapidly accelerate research in leukemia and advance the hunt for treatments, we provide the hallmarks in an online compendium where fellow researchers and oncologists worldwide can build from the resource, tools and findings, LeukemiaAtlas.org.”