Precision medicine therapy developer Denovo Biopharma LLC, announced dosing of the first patient in its biomarker guided Phase III clinical study evaluating the enzastaurin (DB102) in combination with temozolomide and radiation as first line therapy to treat newly-diagnosed glioblastoma multiforme (GBM). The randomized, double-blind, placebo-controlled global study is plans to enroll 300 patients designed to measure is overall survival in patients with Denovo Genomic Marker 1 (DGM1). This GBM study has received Phase III permission from regulatory agencies from US, Canada, and China.
Enzastaurin is an orally available investigational first-in-class small molecule, serine/threonine kinase inhibitor of the PKC beta, PI3K, and AKT pathways that has been studied in more than 3,000 patients across a range of solid and hematological tumor types. Denovo acquired worldwide rights to the candidate from the original developer Eli Lilly. It has received Orphan Drug Designation in diffuse large B-cell lymphoma (DLBCL) and glioblastoma multiforme (GBM) from the FDA and EMA as well as a Fast Track Designation from the FDA last year.
“This GBM study is the second global Phase III trial of DB102 for patients with cancer following our first global Phase III trial of DB102 for patients with diffuse large B-cell lymphoma (DLBCL).,” says Zane Yang, M.D., Denovo’s Chief Medical Officer in a press release. “In both trials, we use DGM1 to identify the patients who will receive the most benefits from DB102 therapy in combination regimen.”
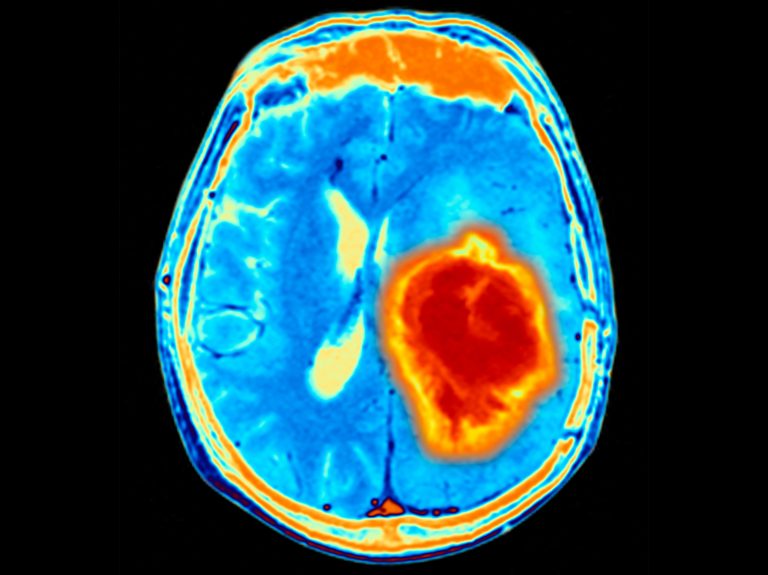
GBM is the most common type of adult primary malignant brain cancer, with 18,000 newly-diagnosed patients in the US and 13,000 deaths annually. Standard treatment for patients with newly diagnosed GBM can include surgery followed by radiation and chemotherapy, but treatment options are limited. The five-year survival rate of patients with GBM is less than five percent.
“GBM remains to be one of the deadliest cancers and the first line drug treatment still relies on temozolomide as the backbone—many promising anticancer drugs, including anti-PD-1 drugs, have failed to improve upon temozolomide’s efficacy,” Yang adds. “I am hopeful that our innovative approach can bring new hope to patients with this difficult-to-treat condition that continues to have a significant unmet need.”
Denovo Biopharma is a clinical-stage biopharmaceutical company that applies biomarker approaches to re-evaluate medicines that have failed in broad patient populations. The company seeks to discover genomic biomarkers correlated with patients’ responses to drug candidates retrospectively. Denovo then designs and executes efficient clinical trials in targeted patient populations to optimize the probability of a successful trial. In additiona to its DB102 trials, the company has six additional late-stage programs including: DB103 (pomaglumetad methionil) for schizophrenia, DB104 (liafensine) for depression, DB105 (formerly ORM-12741) for Alzheimer’s Disease, DB106 (vosaroxin) for acute myeloid leukemia (AML), DB107 (formerly Toca 511 and Toca FC) for recurrent high grade glioma, and DB108 (endostatin) for non-small cell lung cancer (NSCLC).













