Harvard University’s Wyss Institute for Biologically Inspired Engineering plans to commercialize its DNA nanotechnology-based variant-detecting method as a fast, low-cost and multiplexed molecular diagnostic for cancer and infectious diseases, among other indications, through a spinout company launched with $11 million in Series A financing.
NuProbe has inked a worldwide licensing agreement with Harvard’s Office of Technology Development (OTD) to commercialize the technology, developed there and at Rice University by its co-founders. The company said it aims to develop clinical assays capable of simultaneously detecting multiple rare disease-related DNA variants in bodily fluids.
The assays will apply NuProbe’s technology, which is designed to detect rare alleles through DNA toehold probe systems based on molecular competition and precisely-balanced thermodynamics.
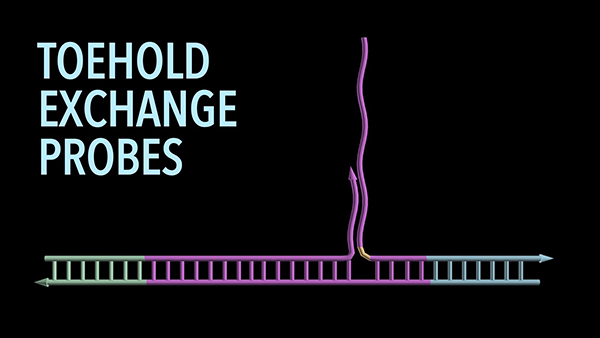
Toehold probes contain two strands of DNA that are hybridized to each other due to complementarity of their nucleotide sequences. The first “probe” strand complements a target sequence, for example, in the human genome, while the second “protector” strand copies part of the target DNA. Toeholds—short sequences at the ends of the probe strand that are either complementary to the target sequence or the protector strand—initiate two exchange reactions.
The toeholds either result in the probe strand being specifically bound to its genomic target DNA, enabling its detection, and the protector strand being released; or the probe strand re-engaging with the protector strand and leaving the target DNA behind. The two competing exchange reactions lead to an equilibrium that is highly predictable and highly sensitive to the presence of a single non-matching nucleotide in the target sequence that could prevent its detection.
NuProbe says its assays will offer specificity and the ability for multiplexing to detect several variants, addressing the length and cost of many present-day tests.
According to preclinical studies cited by the company, its toehold probe designs improve quantitative polymerase chain reaction (qPCR) and next-generation sequencing (NGS) mutation detection by 10 to 100 fold with sensitivity down to 0.01%, and multiplexing capability of 10 to 100 multiplexed reactions with hundreds of mutations in the same test tube. The company says such results can enable detection of non-invasive early stage cancer cell-free DNA in blood plasma.
Toehold probes can also robustly operate at a much broader range of temperatures, the company said, allowing for multiplexing, compared with conventional probes that typically work in more limited temperature settings.
“Toehold probes add new and powerful DNA nanotechnology-driven capabilities to both polymerase chain reaction and next generation sequencing-based methods. Their specificity and robustness may enable clinical research labs to specifically home in on multiple rare genetic variants in a single test with fast turnaround, and in a much more cost-effective way,” said NuProbe co-founder Peng Yin, Ph.D., core faculty member of the Wyss Institute and professor of systems biology at Harvard Medical School.
Dr. Yin co-developed the toehold probes with his former postdoctoral fellow David Zhang, Ph.D., a NuProbe co-founder who is now the Ted Law Jr. assistant professor of bioengineering at Rice University.
“We have validated our technology by identifying hundreds of single nucleotide variants in samples including cell-free DNA extracted from blood of cancer patients,” Dr. Zhang stated. NuProbe said it validated the technology with samples from U.S. patients, and is conducting preliminary clinical trials in China.
“With the robustness and versatility of our method, we potentially could facilitate personalized diagnostics in the future also through the use of more portable and economical PCR instruments with lower temperature accuracy and uniformity in many clinical settings,” Dr. Zhang added.
Another co-founder of NuProbe is Victor Shi, Ph.D., an investor in the company who is a former founding president of Qiagen Asia Pacific and a former faculty member at National University of Singapore School of Medicine. Two former postdoctoral fellows working on Dr. Yin’s Wyss Institute research team will join the startup, Jongmin Kim, Ph.D., and Nicolas Garreau, Ph.D.
NuProbe said it will continue development of ultra-sensitive, non-invasive blood tests for early detection of cancer and infectious diseases using proceeds of its $11 million Series A financing. The funding will also be used to grow NuProbe’s staff in its Cambridge, Mass. and Shanghai, China offices, as well as continue technology development, clinical trials and commercialization.
Sequoia China and Serica Partners co-led the financing, with participation by additional investors that included WuXi AppTec Corporate Ventures.











