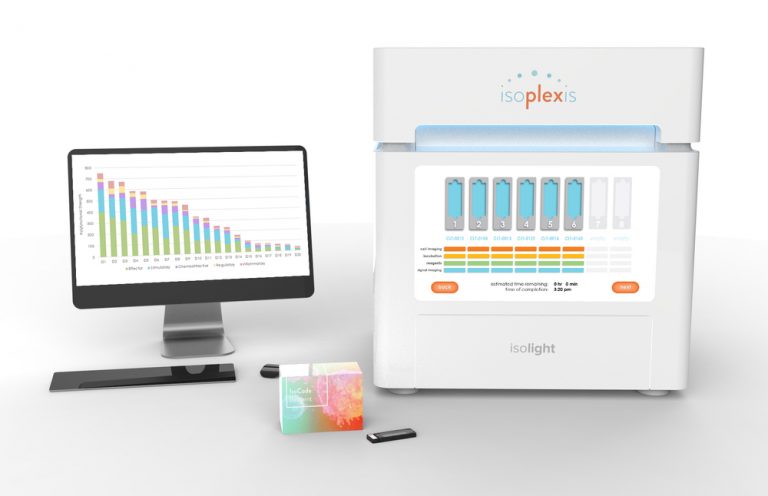
IsoPlexis has won a $4 million grant from the National Cancer Institute (NCI)’s Small Business Innovation Research (SBIR) Development Center toward commercializing its IsoLight single-cell, secreted protein analysis system.
Based in Branford, CT, IsoLight is a precision engineering platform designed for research use only in CAR-T therapy product evaluation, quality check pre-infusion, and correlative studies to understand and characterize differences among single cells. The system maps thousands of cells per sample, with the goal of revealing full functional profiles and polyfunctionality among cell subsets to determine patient response and product quality.
“In terms of the overall goals of the project, it is specifically to translate our findings—whether it be from a protocol or a sample analysis perspective—to laboratories worldwide, to be that last leg as we translate this outside of our own company to everyone else who’s now using this on the IsoLight externally,” Sean Mackay, IsoPlexis CEO and Co-Founder, told Clinical OMICs.
Mackay added that the new funding demonstrated IsoPlexis’ leadership in, and commitment to, advancing the future of next generation CAR-T and other engineered T-cell therapies in blood cancers, solid tumor, and beyond.
Using the patented consumable IsoCode® Chip (formerly called a single-cell barcode chip or SCBC), onto which a sample is loaded, IsoLight is designed to capture single-cell, secretomic, cytokine profiles from thousands of single cells in parallel to better understand complex immunotherapy patient response. IsoLight can accommodate 1 to 8 chips and reagent cartridges per run, with a throughput of 1,000 to 8,000 single cells.
The IsoCode chip, integrated with IsoPlexis’ automated IsoSpeak Informatics platform, can simultaneously measure up to 32 cytokines secreted per single cell. The technology is designed to help discover new patient relationships among heterogeneous cells, as well as clearly define subsets of powerful, multi-functional protein secreting cells that can help predict patient outcome and determine disease progression.
IsoPlexis says the unique benefit of its IsoLight technology is its ability to reveal highly multiplexed, true cytokine secretion from a thousand single cells in parallel. That capability, in turn, can unlock uniquely correlative biomarkers of response and revealing mechanism of CAR-T therapies.
Last year, IsoLight uniquely predicted responses of CAR engineered T cell therapy patients in blood cancers, pre-therapy, for the first time. IsoPlexis customers also released multiple data sets defining the quality of patient response of CAR-T, TCR-T, CRISPR edited cell therapies, and more. Those customers ranged from Kite, a Gilead Company, to institutions that included MD Anderson Cancer Center, Moffitt Cancer Center, and City of Hope
IsoPlexis’ announcement of the $4 million grant comes a month after the company said it raised $25 million in Series C financing, with the capital to be used in part toward the launch of new single-cell immune biomarker applications intended to address high-need areas based on data generated and published on IsoPlexis’ platform.
Those launches, according to the company, include single-cell polyfunctional inflammation solutions targeted towards neuroinflammation and autoimmunity, single-cell solutions for innate and myeloid cell types that contribute to immunotherapy response, single-cell energy state assays focused on advanced metabolomics in oncology, and tumor resistance assays focused on advanced tumor signaling.
IsoPlexis added that the Series C funding will also be used toward expanding its global operational, manufacturing, and commercial team across US, Europe and Asia. That expansion will add to the company’s workforce, which stood last month at 105 employees.
“We’ve already hired quite a few people especially in the commercial support and commercial operations and sales functions.
According to Mackay, IsoPlexis sees three avenues for expanding its operations. The main avenue is customer support/protocol support, the upstream requirement for people who already purchased, and are in the purchasing process for the IsoLight system. That will require application scientists, and service team and service technicians.
The second avenue, he said, is international support of customers in Europe and China. In August 2018, Tekon Biotech, a leading instrumentation distributor, agreed to distribute IsoPlexis’ products in China through an exclusive agreement whose value was not disclosed. The company hopes to capitalize on China’s growth in CAR-T clinical trials, to which it credits government programs ranging from the Institute of Biophysics’ “One-Three-Five” grant initiative to the “Made in China 2025” industrial policy aimed at catapulting China to global leadership in high-tech manufacturing.
The third and final area for growth, Mackay said, was continued development of the application.
“We have in-licensed technologies from Yale (University) and CalTech, and other institutions in terms of continuing to move beyond CAR-T cell analysis, even T-cell analysis with checkpoint inhibitors in the blood, and moving into innate myeloid cells. That’s a big push for us this year,” Mackay said. “We’re also looking at autoimmune diseases, and diseases of the CNS. That’s a big push for us.”
The $25 million financing round was led by Northpond Ventures and included new strategic investment along with current investors Spring Mountain Capital, Ironwood Capital, North Sound Capital, and Connecticut Innovations, the Nutmeg State’s strategic venture capital arm.











